|
|
|
|
||||||||
|
|
||||||||||
|
The
Journal of Physiology (1999), 515.2,
pp. 439-452
© Copyright 1999 The Physiological Society
Glucose-receptive neurones in the rat ventromedial hypothalamus express KATP
channels composed of Kir6.1 and SUR1 subunits
K. Lee, A. K. Dixon, P. J. Richardson * and R. D. Pinnock
Parke Davis Neuroscience Research Centre, Cambridge University Forvie
Site, Cambridge CB2 2QB and * Department of Pharmacology, Tennis Court Road,
Cambridge CB2 1QJ, UKMS 8753 Received 25 September 1998;
accepted after revision 23 November 1998.
|
|
ABSTRACT |
|
|
- Patch-clamp recordings were
made from rat ventromedial hypothalamic neurones in slices of brain tissue
in vitro. In cell-attached recordings, removal of extracellular
glucose or metabolic inhibition with sodium azide reduced the firing rate
of a subpopulation of cells through the activation of a 65 pS channel that
was blocked by the sulphonylureas tolbutamide and glibenclamide.
- In whole-cell patch-clamp
recordings, in the absence of ATP in the electrode solution,
glucose-receptive neurones gradually hyperpolarized due to the induction
of an outward current at -60 mV. This outward current and the resultant
hyperpolarization were blocked by the sulphonylureas tolbutamide and
glibenclamide.
- In recordings where the
electrode solution contained 4 mM ATP, this outward current was not
observed. Under these conditions, 500 µM diazoxide was found to induce an
outward current that was blocked by tolbutamide.
- In cell-attached recordings
diazoxide and the active fragment of leptin (leptin 22-56) reduced the
firing rate of glucose-receptive neurones by the activation of a channel
with similar properties to that induced by removal of extracellular
glucose.
- Reverse transcription
followed by the polymerase chain reaction using cytoplasm from single
glucose-receptive neurones demonstrated the expression of the ATP-sensitive
potassium (KATP) channel subunits Kir6.1 and SUR1 but not
Kir6.2 or SUR2.
- It is concluded that
glucose-receptive neurones within the rat ventromedial hypothalamus
exhibit a KATP channel current with pharmacological and
molecular properties similar to those reported in other tissues.
|
|
INTRODUCTION |
|
|
It is well established that the
ventromedial hypothalamus (VMH) is an important centre involved in the
monitoring of glucose status and the regulation of feeding (Anand et al.
1964; Dunn-Meynell et al. 1997). The ability of the VMH to respond to
extracellular glucose levels is thought to arise due to the presence of
glucose-receptive neurones within this nucleus (Ono et al. 1982). These
neurones respond to changes in extracellular glucose as a result of ATP-sensitive
potassium (KATP) channel expression (Ashford et al. 1990a).
This group of potassium channels
has been well characterized in peripheral tissues and the channels have been
found in many types of excitable cell where they act to link cell excitability
with metabolic status (Ashcroft & Ashcroft, 1990). In addition to their
regulation by intracellular ATP, various pharmacological agents modulate KATP
channel activity. For example, potassium channel openers such as diazoxide
potentiate channel activity (Kozlowski et al. 1989) whilst agents
including the antidiabetic sulphonylureas (Sturgess et al. 1985) and
imidazoline compounds (Dunne, 1991) inhibit channel activity.
In contrast to most forms of KATP
channel, the VMH KATP channel is reported to display several
features which distinguish it from other types of KATP channel. For
example, this channel is reported to exhibit a large unitary conductance (150
pS under symmetrical conditions; Ashford et al. 1990a; but see
Routh et al. 1997) and has been shown to be less sensitive to ATP in
excised inside-out patches (half-maximal inhibitory concentration of 3 mM
compared with 12-20 µM in pancreatic ![]() cells).
cells).
Importantly, the pharmacological
properties of this channel are poorly understood and this factor has greatly
hindered its more extensive investigation. Although it has been reported that
this channel can be inhibited by the sulphonylurea tolbutamide in cell-attached
patch recordings, this sensitivity is lost following patch excision indicating
a novel form of coupling between the channel and its associated sulphonylurea
receptor (Ashford et al. 1990b). Furthermore, it has been
reported that although the channel itself is insensitive to glibenclamide, this
agent can block the inhibitory effects of tolbutamide.
In view of the complex nature with
which this channel interacts with the sulphonylureas, the present study was
initiated to examine the properties of this channel in more detail. In contrast
to previous studies, our findings suggest that glucose-receptive neurones in
the VMH display KATP channel characteristics similar to those found
in the pancreatic ![]() cell.
cell.
|
|
METHODS |
|
|
Preparation
Male Sprague-Dawley rats (14-28
days old) were killed by cervical dislocation, their brains were removed and
300 µm coronal slices containing the VMH were prepared in ice-cold
physiological saline using a vibratome. A Zeiss Axioskop microscope (Carl Zeiss
Ltd, Welwyn Garden City, UK) fitted with a × 64 water-immersion objective lens
was used to view slices (see Stuart et al. 1993).
Recording and analysis
Recording electrodes were pulled
from borosilicate glass capillaries and when filled with electrolyte had
resistances of 8-12 M![]() for isolated patch experiments, and 3-6 M
for isolated patch experiments, and 3-6 M![]() for whole-cell recording. Electrophysiological signals were detected using an
Axopatch-1D patch-clamp amplifier (Axon Instruments Inc.) and were recorded
onto digital audio tape. Following formation of the whole-cell configuration,
series resistance was partially compensated using the amplifier and cellular
conductance was continually monitored via the injection of hyperpolarizing
current (current-clamp mode; -50 pA in amplitude, 300 ms in duration at 0·1 Hz)
or voltage (voltage-clamp mode; -10 mV in amplitude, 300 ms duration at 0·1
Hz). Membrane signals were filtered at 1 kHz for whole-cell experiments and at
500 Hz for single channel recordings. These data were digitized at 5 kHz
through a Digidata 1200B A/D converter using pCLAMP 6.0.3 software (Axon
Instruments Inc.). In voltage-clamp recordings, neurones were clamped at -60 mV
and current-voltage relationships were examined using a voltage ramp protocol
between -140 and -60 mV (20 mV s-1).
for whole-cell recording. Electrophysiological signals were detected using an
Axopatch-1D patch-clamp amplifier (Axon Instruments Inc.) and were recorded
onto digital audio tape. Following formation of the whole-cell configuration,
series resistance was partially compensated using the amplifier and cellular
conductance was continually monitored via the injection of hyperpolarizing
current (current-clamp mode; -50 pA in amplitude, 300 ms in duration at 0·1 Hz)
or voltage (voltage-clamp mode; -10 mV in amplitude, 300 ms duration at 0·1
Hz). Membrane signals were filtered at 1 kHz for whole-cell experiments and at
500 Hz for single channel recordings. These data were digitized at 5 kHz
through a Digidata 1200B A/D converter using pCLAMP 6.0.3 software (Axon
Instruments Inc.). In voltage-clamp recordings, neurones were clamped at -60 mV
and current-voltage relationships were examined using a voltage ramp protocol
between -140 and -60 mV (20 mV s-1).
To assess whether a particular
procedure led to a significant change in the magnitude of the current under
study, data were subjected to Student's t test. All data in the text and
figures are presented as mean values ±
Solutions and drugs
The physiological saline contained
(mM): 125·0 NaCl, 25·0 NaHCO3, 10·0 glucose, 2·5 KCl, 1·25 NaH2PO4,
2·0 CaCl2 and 1·0 MgCl2, and was bubbled with a 95 % O2-5
% CO2 gas mixture. In some experiments, glucose was removed from the
physiological saline and was replaced with 10 mM sucrose to maintain
osmolarity. The intracellular (pipette) solution was adjusted to pH 7·4 using
KOH in all experiments. For whole-cell recordings this solution comprised (mM):
120·0 potassium gluconate, 10·0 NaCl, 2·0 MgCl2, 0·5 K2EGTA,
10·0 Hepes, 4·0 Na2ATP and 0·1 Na2GTP. In some
experiments, Na2ATP was removed from this solution. For subsequent
epifluorescence examination of cell morphology, 1 mg ml-1 Lucifer
Yellow was added to the pipette solution used to dialyse some cells. Slices
were fixed with 4 % paraformaldehyde before being cleared in DMSO and viewed
whole mount (Grace & Llinas, 1985). In cell-attached and outside-out patch
experiments, the electrode solution comprised (mM): 120·0 potassium gluconate,
0·5 K2EGTA, 0·5 MgCl2 and 10·0 Hepes.
Throughout the course of an
experiment, slices were continuously perfused at a rate of 3-4 ml min-1
with physiological saline by means of a gravity feed system. Tolbutamide and
other drugs used in this study were applied by changing the solution which
superfused the slice to one which contained the drug. In voltage-clamp
recordings, 1 µM TTX, 50 µM D-2-amino-5-phosphovalerate (D-APV) and 1 µM
2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo(f) quinoxalone (NBQX) were usually
added to the physiological saline. All experiments were conducted at 32-35°C.
All drugs were obtained from Sigma
except NBQX and D-APV which were from Tocris Cookson (Bristol, UK) and the
active fragment of leptin (leptin 22-56 (Powis et al. 1998)) which was
from Bachem (Saffron Walden, UK).
Single cell RT-PCR
Analysis of gene expression in
single neurones was achieved as described previously (Lee et al. 1998).
Briefly, at least 40 % of the somatic cytoplasm from single neurones was
aspirated into the recording electrode which was subsequently withdrawn from
the cell forming an outside-out patch. The contents of the electrode were
forced into a microtube and the RNA reverse transcribed using an anchored
oligo-dT primer and 200 U of Moloney murine leukaemia virus (MMLV) reverse
transcriptase (BRL) according to the manufacturer's recommendations. After 60
min at 37°C the cDNA was stored frozen at -20°C prior to processing. After
amplification of the cDNA using Taq polymerase (Dixon et al.
1998), the expression of specific genes was measured using primers designed to
amplify products of between 120 and 250 base pairs in length, close to the 3'
ends of the mRNA transcripts.
The primers used were as follows.
Kir6.1 (accession number D42145): forward primer (bases 1292-1311),
GAGTGAACTGTCGCACCAGA; reverse primer (bases 1539-1520), CGATCACCAGAACTCAGCAA.
Kir6.2 (accession number X97041): forward primer (bases 787-804),
TCCAACAGCCCGCTCTAC; reverse primer (bases 954-937), GATGGGGACAAAACGCTG.
Sulphonylurea receptor 1 (SUR1; accession number L40624): forward primer (bases
4824-4842), TGAAGCAACTGCCTCCATC; reverse primer (bases 5005-4987),
GAAGCTTTTCCGGCTTGTC. Sulphonylurea receptor 2 (SUR2; accession number D83598):
forward primer (bases 4853-4872), ACCTGCTCCAGCACAAGAAT; reverse primer (bases
4997-4976), TCTCTTCATCACAATGACCAGG. ![]() -Tubulin
(accession number V01226): forward primer (bases 300-318), CACTGGTACGTGGGTGAGG;
reverse primer (bases 471-450): TTTGACATGATACAGGGACTGC. Cytochrome oxidase
(accession number L48209): forward primer (bases 96-116), ATCACCATTGGGCTCACTTC;
reverse primer (bases 281-264), ATCCCAGGGTAAGCCAGC. Synaptotagmin 1 (accession
number X52772): forward primer (bases 4022-4042), AGGGGCTTTCCTATCTAAGGG;
reverse primer (bases 4223-4204), GTTGGCAGTGTTGCAAGAGA. Leptin receptor (ObRb;
accession number D84551): forward primer (bases 3301-3320),
CCATTCCCAGCTCACTGTCT; reverse primer (bases 3437-3418), GAACAGGATTGAAACTGGGG.
These PCR reactions were run for 45 cycles of 92°C (denaturing, 2·5 min), 55°C
(annealing, 1·5 min) and 72°C (extension, 1 min), followed by a final extension
of 10 min at 72°C. The PCR products were separated on 2·5 % agarose gels and
the product sizes were as predicted from the sequences. Confirmation of the
sensitivity and specificity of these PCR reactions was achieved as described
previously (Lee et al. 1998; Dixon et al. 1998). The nature of
the Kir6.1 and SUR1 products was confirmed by sequencing of the amplified PCR
products on an ABI 310 sequencer (Applied Biosystems, Warrington, UK).
-Tubulin
(accession number V01226): forward primer (bases 300-318), CACTGGTACGTGGGTGAGG;
reverse primer (bases 471-450): TTTGACATGATACAGGGACTGC. Cytochrome oxidase
(accession number L48209): forward primer (bases 96-116), ATCACCATTGGGCTCACTTC;
reverse primer (bases 281-264), ATCCCAGGGTAAGCCAGC. Synaptotagmin 1 (accession
number X52772): forward primer (bases 4022-4042), AGGGGCTTTCCTATCTAAGGG;
reverse primer (bases 4223-4204), GTTGGCAGTGTTGCAAGAGA. Leptin receptor (ObRb;
accession number D84551): forward primer (bases 3301-3320),
CCATTCCCAGCTCACTGTCT; reverse primer (bases 3437-3418), GAACAGGATTGAAACTGGGG.
These PCR reactions were run for 45 cycles of 92°C (denaturing, 2·5 min), 55°C
(annealing, 1·5 min) and 72°C (extension, 1 min), followed by a final extension
of 10 min at 72°C. The PCR products were separated on 2·5 % agarose gels and
the product sizes were as predicted from the sequences. Confirmation of the
sensitivity and specificity of these PCR reactions was achieved as described
previously (Lee et al. 1998; Dixon et al. 1998). The nature of
the Kir6.1 and SUR1 products was confirmed by sequencing of the amplified PCR
products on an ABI 310 sequencer (Applied Biosystems, Warrington, UK).
|
|
RESULTS |
|
|
Identification of
glucose-receptive neurones in the VMH
Initial identification of
glucose-receptive neurones within the VMH was achieved using cell-attached
patch recordings. In preliminary studies 12 out of 37 neurones within the VMH
were identified as glucose receptive by virtue of the reduction in firing rate
that was observed in response to removal of extracellular glucose (Fig. 1). Within 5
min of glucose removal, the firing rate of these neurones had reduced from 3·2
± 0·9 to 1·5 ± 0·5 Hz (n = 9 observations). This reduction in firing
rate continued until complete cessation of action potential firing was achieved
15-30 min after glucose removal. In each case, this decrease in firing rate was
associated with the appearance of a single channel current 3·8 ± 0·7 pA in
amplitude (at 0 mV pipette potential) with a channel open probability of 0·3 ±
0·1 (Fig. 2A;
n = 8). The current was observed to reverse polarity at a pipette potential
between 60 and 80 mV and exhibited a unitary conductance of 65·4 ± 5·6 pS (as
judged over the linear part of the I-V relationship; Fig. 2C, n
= 8). The activity of this channel was unaffected by membrane voltage (Fig. 2B) but
was completely and reversibly inhibited by bath application of 200 µM
tolbutamide (Fig. 2A;
n = 7) or the re-addition of 10 mM glucose to the bath solution (n =
6).
|
|
|
View larger version |
|
|
||
|
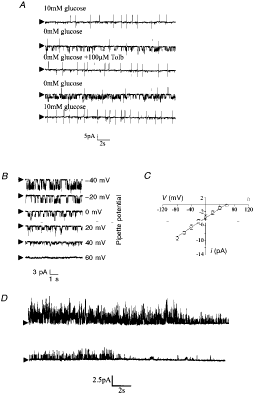
Figure 1.
Cell-attached recording illustrating the effect of aglycaemia on the firing
properties of a glucose-receptive neurone Note that within 2 min of the onset of glucose
removal a change in firing rate is observed, gradually resulting in complete
cessation of firing after a further 15-20 min. This process was associated
with the activation of a 3·3 pA channel at this pipette potential (0 mV).
Tolb, tolbutamide. |
||
|
|
|
View larger version |
|
|
||
|
Figure 2.
Single channel characteristics of the tolbutamide-sensitive channel activated
by aglycaemia A, cell-attached recording made at a
pipette potential of 0 mV. Removal of glucose from the bath led to the
activation of two 3·7 pA channels which were inhibited by tolbutamide.
Arrowheads mark closed state. B, cell-attached recording of the
aglycaemia-activated channel at different pipette potentials. C, current-voltage
relationship revealed a unitary conductance of 65·4 ± 5·6 pS in the linear
part of the plot (denoted by the line). Each point is the mean of between
three and five observations. D, subsequent formation of an outside-out
patch held at 0 mV under quasi-physiological ionic conditions (the electrode
contained 120·0 mM potassium gluconate, 0·5 mM K2EGTA, 0·5 mM MgCl2
and 10·0 mM Hepes whilst physiological saline was in the bath) resulted in
the rapid loss of channel activity. |
||
To examine the properties of this
channel in more detail, further single channel analysis was attempted after the
formation of outside-out patches. Unfortunately, as shown in Fig. 2D,
following patch excision there was a rapid loss of channel activity (run-down, n
= 5) which precluded further study.
Due to the long time interval between
removal of extracellular glucose and channel activation, in further experiments
1 mM NaN3 was used in order to inhibit VMH neurones metabolically.
In these experiments 8 out of 25 neurones were found to respond with a
reduction in action potential firing rate and the opening of a channel with
characteristics indistinguishable from those observed above (Fig. 3). As found
previously, the activity of this channel was completely inhibited by 200 µM
tolbutamide (Fig. 3A).
Furthermore, in four separate experiments, this channel was found to be
inhibited by the application of 100 or 200 nM glibenclamide to the bath
solution. However, unlike tolbutamide, the effects of this second generation
sulphonylurea were not reversed on continued washing (Fig. 3B).
|
|
|
View larger version |
|
|
||
|
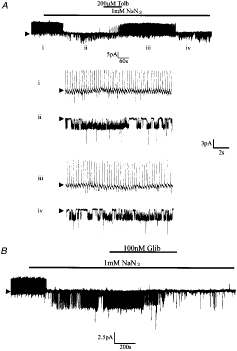
Figure 3. The
effect of metabolic inhibition with NaN3 on the electrical
characteristics of glucose-receptive neurones A, cell-attached recording made at a
pipette potential of 0 mV. Bath application of NaN3 induced the
activity of a tolbutamide-sensitive 3·5 pA channel. The numerals refer to the
traces shown on an expanded time scale in the lower part of the figure. B,
cell-attached recording at 0 mV illustrating the effect of 100 nM
glibenclamide (Glib) on the NaN3-activated channel. |
||
In contrast to these findings, in
control experiments performed on seven VMH neurones whose activity was not
altered by removal of extracellular glucose or metabolic inhibition with NaN3,
neither tolbutamide nor glibenclamide was found to affect action potential
firing rate or background channel activity (not shown).
Identification of KATP
channel activity
When VMH neurones were dialysed
with an ATP-free solution and glucose was removed from the physiological
saline, 86 out of 234 neurones underwent a gradual hyperpolarization with
cessation of action potential firing and an associated decrease in apparent
input resistance. The initial resting membrane potential of these neurones was
-49·4 ± 3·2 mV (n = 42) and the input resistance was 395 ± 43·2 M![]() (n = 25). At this membrane potential, these cells were found to fire
spontaneous action potentials at a rate of 1-4 Hz. The amplitude of these
action potentials was 72·5 ± 2·9 mV (n = 27) whilst the amplitude of the
associated after-hyperpolarization was 17·3 ± 1·2 mV (n = 27).
Morphological analysis of Lucifer Yellow-filled cells revealed that these
neurones were usually multipolar with three or more primary dendrites extending
from the soma (n = 8/12). Furthermore, this analysis revealed these
cells to be located towards the upper and lateral borders of the VMH.
(n = 25). At this membrane potential, these cells were found to fire
spontaneous action potentials at a rate of 1-4 Hz. The amplitude of these
action potentials was 72·5 ± 2·9 mV (n = 27) whilst the amplitude of the
associated after-hyperpolarization was 17·3 ± 1·2 mV (n = 27).
Morphological analysis of Lucifer Yellow-filled cells revealed that these
neurones were usually multipolar with three or more primary dendrites extending
from the soma (n = 8/12). Furthermore, this analysis revealed these
cells to be located towards the upper and lateral borders of the VMH.
Within approximately 10 min of
membrane breakthrough, these neurones hyperpolarized from their initial resting
membrane potential to -71·6 ± 5·2 mV (n = 42). This hyperpolarization
was associated with a marked reduction in apparent input resistance (to 123·5 ±
12·3 M![]() ;
n = 42, Fig. 4A).
;
n = 42, Fig. 4A).
|
|
|
View larger version |
|
|
||
|
Figure 4. The
effect of dialysis with an ATP-free electrode solution A, continuous whole-cell current-clamp
recording demonstrating the effect of tolbutamide on the hyperpolarization
resulting from dialysis with an ATP-free electrode solution. The effects of
tolbutamide were retained in the presence of TTX. B, continuous
whole-cell current-clamp recording illustrating the effect of glibenclamide
on membrane properties. |
||
Bath application of the
sulphonylurea tolbutamide at this point rapidly (within 2 min) depolarized
neurones (by 19·5 ± 3·9 mV; n = 21) with a concomitant increase in
apparent input resistance (to 281·9 ± 11·1 M![]() ;
n = 21) and return of action potential firing (Fig. 4A).
These effects of tolbutamide were reversible on washout. The ability of
tolbutamide to depolarize these cells and to increase input resistance
persisted after treatment of the slice with 1 µM TTX (control, 18·1 ± 4·5 mV
depolarization; 1 µM TTX, 18·2 ± 4·1 mV depolarization; n = 5).
;
n = 21) and return of action potential firing (Fig. 4A).
These effects of tolbutamide were reversible on washout. The ability of
tolbutamide to depolarize these cells and to increase input resistance
persisted after treatment of the slice with 1 µM TTX (control, 18·1 ± 4·5 mV
depolarization; 1 µM TTX, 18·2 ± 4·1 mV depolarization; n = 5).
In contrast to previous findings
(Ashford et al. 1990b) all neurones found to respond in this
manner were also sensitive to the second generation sulphonylurea glibenclamide
(n = 15; Fig.
4B). Bath application of 50-100 nM glibenclamide induced a slow
(taking 3-5 min) membrane depolarization 22·3 ± 5·2 mV (n = 15) in
magnitude that was associated with a concomitant increase in apparent input
resistance (from 111·9 ± 12·1 M![]() (n = 21) to 312·9 ± 14·2 M
(n = 21) to 312·9 ± 14·2 M![]() (n = 15)). Glibenclamide was found to exert similar effects in the
presence of 1 µM TTX (n = 5, not shown).
(n = 15)). Glibenclamide was found to exert similar effects in the
presence of 1 µM TTX (n = 5, not shown).
To determine the nature of the
sulphonylurea-sensitive current(s) responsible for these effects, neurones were
voltage clamped at -60 mV. At this potential, an outward current of 122·3 ±
25·3 pA (n = 28) was observed to develop over time when ATP was omitted
from the electrode solution (Fig. 5A). The
development of this outward current exhibited a similar time course to the
hyperpolarization seen in current-clamp recordings and was associated with an
increase in cellular conductance (from 4·5 ± 0·5 to 9·2 ± 0·7 nS as assessed by
10 mV hyperpolarizing step commands (n = 5) from -60 mV). Using
depolarizing ramps from -140 to -60 mV this time-dependent outward current was
found to exhibit a reversal potential of -98·5 ± 3·9 mV (n = 12) under
the present ionic conditions (Fig. 5B).
|
|
|
View larger version |
|
|
||
|
Figure 5. The
effect of glibenclamide on the time-dependent outward current at a holding
potential of -60 mV A, the large deflection at the start of the
recording indicates formation of the whole-cell configuration. The regular
downward pulses are current responses to -10 mV steps whilst the larger
labelled deflections are the current responses to voltage ramps from -140 to
-60 mV as depicted in B. B, expanded traces of the current responses
to the voltage ramps shown in A. The numerals beside these currents
correspond to those shown in A. |
||
Once this time-dependent current
had reached its peak magnitude, bath application of either tolbutamide (200 µM)
or glibenclamide (100 nM) was found to inhibit this current (Fig. 5A).
However, although the inhibitory effects of tolbutamide were reversible on
washout, the inhibition produced by glibenclamide was not reversed during the
time course of these experiments. The ability of both tolbutamide and
glibenclamide to inhibit this current was unaffected by pre-application of the
glutamate receptor antagonists D-APV (50 µM) and NBQX (1 µM), or by the use of
a Ca2+-free artificial cerebrospinal fluid (ACSF) containing 10 mM
MgCl2 in conjunction with D-APV and NBQX, thus discounting any
possible presynaptic effects (Fig. 5A).
In a small number of experiments
neurones identified as glucose receptive using cell-attached patch recordings
were subsequently re-patched using whole-cell electrodes containing a pipette
solution devoid of ATP. In each case, these neurones gradually underwent a
hyperpolarization that was indistinguishable from that described above and
which was readily reversed by application of 200 µM tolbutamide (n = 4,
not shown). Furthermore, three cells which were characterized as non-glucose
receptive using cell-attached recording criteria did not undergo such a
sequence of events when susequently re-patched using ATP-free whole-cell
electrodes.
Pharmacology of the KATP
channel current
Since previous studies report that
the KATP channel current present in VMH neurones differs from that
found in the periphery, we examined the effects of several potassium channel
inhibitors on the sulphonylurea-sensitive current that develops when
glucose-receptive neurones are held at -60 mV and ATP is removed from the
electrode solution. (see Fig. 6).
|
|
|
View larger version |
|
|
||
|
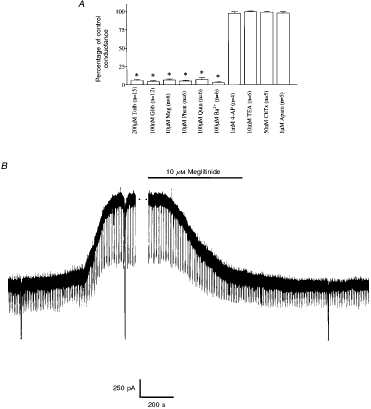
Figure 6.
Pharmacology of the sulphonylurea-sensitive current A, mean data illustrating the pharmacology
of the sulphonylurea-sensitive current at -60 mV. Control conductance was
measured as the difference between five consecutive -10 mV voltage steps
immediately following membrane breakthrough and after complete activation of
the KATP channel current. Each value is the mean of the number of
observations indicated in parentheses. Error bars indicate |
||
Under these conditions this current
was completely and reversibly inhibited by application of the non-specific
potassium channel inhibitor BaCl2 (100 µM). Similarly, this
conductance was sensitive to bath application of quinine (100 µM) although
neither 10 mM TEA+ nor 1 mM 4-aminopyridine (4-AP) was found to
exert any significant effect on this current (Fig. 6A).
In addition to tolbutamide and the
second generation sulphonylurea glibenclamide, the non-sulphonylurea structural
component of glibenclamide, HB699 or meglitinide (Lee et al. 1994a),
also inhibited this current in a poorly reversible manner (Fig. 6B).
Similarly, the imidazoline compound phentolamine (Fig. 6A;
Dunne, 1991) inhibited this current in a manner that was not readily reversible
on continued washing. In contrast to the inhibition observed with the above
compounds the Ca2+-activated potassium channel inhibitors
charybdotoxin and apamin were both without effect on this current in these
cells (Fig. 6A).
Potassium channel openers
In cell-attached recordings the
effect of 500 µM diazoxide was tested in seven glucose-receptive neurones. This
potassium channel opener reduced the rate of action potential firing via the
activation of a tolbutamide-sensitive channel with properties indistinguishable
from those observed above. In contrast, 500 µM diazoxide was without
significant effect in five cells that were insensitive to glucose. These
effects of diazoxide on glucose-receptive neurones were mimicked by bath
application of the physiologically active fragment of leptin (leptin 22-56, 50
nM; n = 3; Fig.
7). However, in contrast the effects of this peptide took longer to develop
and were not readily reversed.
|
|
|
View larger version |
|
|
||
|
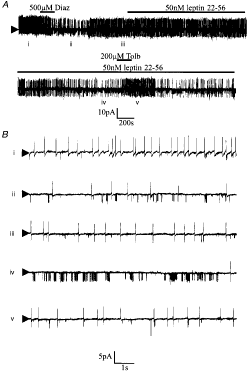
Figure 7. The
effect diazoxide and the active fragment of leptin on the electrical
characteristics of a glucose-receptive neurone A, cell-attached recording made at a
pipette potential of 0 mV. Bath application of diazoxide or leptin 22-56
induced the activity of a tolbutamide-sensitive 3·5 pA channel. The numerals
refer to the traces shown on an expanded time scale in B. |
||
In whole-cell recordings in the
presence of 4 mM Na2ATP, no sulphonylurea-sensitive
hyperpolarization was observed to develop with time in any of the VMH neurones
tested (n = 35). Under these conditions, bath application of 500 µM
diazoxide hyperpolarized 12 of the neurones tested (by 14·1 ± 3·9 mV; n
= 12, Fig. 8) in
a manner that was unaffected by treatment of the slice with 1 µM TTX (n
= 5) but was blocked by co-application of tolbutamide (200 µM, n = 3,
not shown).
|
|
|
View larger version |
|
|
||
|
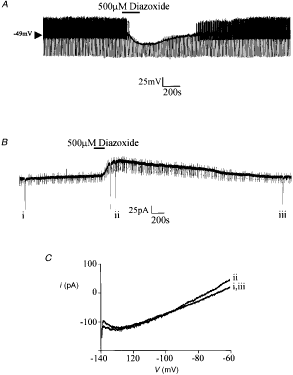
Figure 8. The
effect of diazoxide on VMH glucose-receptive neurones A, in the presence of 4 mM ATP in the
electrode, diazoxide induces a membrane hyperpolarization with an associated
decrease in input resistance. B, in a neurone voltage clamped at -60
mV, diazoxide induces an outward current. The regular downward pulses are
current responses to -10 mV steps whilst the large deflections are the
current responses to voltage ramps from -140 to -60 mV as depicted in C.
C, expanded traces of the current responses to the voltage ramps shown in
B. The numerals beside these currents correspond to those shown in B.
|
||
Similarly, with 4 mM Na2ATP
in the electrode solution, no time-dependent outward current was observed in
neurones voltage clamped at -60 mV. However, bath application of diazoxide
induced an outward current, 89·3 ± 32·4 pA in magnitude (n = 9, Fig. 8B),
which was associated with an increase in cellular conductance (from 4·2 ± 0·6
to 8·9 ± 0·5 nS as assessed by 10 mV hyperpolarizing step commands; n =
5) and had a reversal potential of -99·2 ± 4·4 mV with 2·5 mM K+
present in the extracellular solution (n = 5, not shown). In contrast,
when diazoxide was applied concomitantly with 200 µM tolbutamide, this outward
current was not seen to develop (n = 3, not shown). The effects of
diazoxide were not mimicked by the potassium channel openers pinacidil (500 µM,
n = 4) or levcromakalim (500 µM, n = 4).
Single cell RT-PCR studies
The results presented so far
suggest that the channel responsible for conferring glucose sensitivity upon
these VMH neurones has a similar sulphonylurea and diazoxide sensitivity to the
KATP channel found in pancreatic ![]() cells
(Ashcroft & Ashcroft, 1990). Recent studies have shown that these channels
are formed by the molecular interaction between an inwardly rectifying K+
channel subunit (Kir 6.1 or Kir 6.2; Inagaki et al. 1995b; Sakura
et al. 1995) and a high affinity receptor for the sulphonylureas (SUR1 or
SUR2; Inagaki et al. 1995a). To examine whether the KATP
channel present in these glucose-receptive neurones was formed from the same
subunits the cytoplasm of six cells identified as glucose responsive under
whole-cell recording conditions was harvested and subjected to reverse
transcription. The resulting cDNA was subsquently amplified and subjected to
PCR analysis using primer oligonucleotides specific for the housekeeper genes synaptotagmin
1, cytochrome oxidase and
cells
(Ashcroft & Ashcroft, 1990). Recent studies have shown that these channels
are formed by the molecular interaction between an inwardly rectifying K+
channel subunit (Kir 6.1 or Kir 6.2; Inagaki et al. 1995b; Sakura
et al. 1995) and a high affinity receptor for the sulphonylureas (SUR1 or
SUR2; Inagaki et al. 1995a). To examine whether the KATP
channel present in these glucose-receptive neurones was formed from the same
subunits the cytoplasm of six cells identified as glucose responsive under
whole-cell recording conditions was harvested and subjected to reverse
transcription. The resulting cDNA was subsquently amplified and subjected to
PCR analysis using primer oligonucleotides specific for the housekeeper genes synaptotagmin
1, cytochrome oxidase and ![]() -tubulin
together with the known KATP channel subunits (Kir6.1, Kir6.2, SUR1
and SUR2) and the long form of the leptin receptor (Ob-Rb). In each
glucose-receptive neurone tested, mRNA for the three housekeeping genes and the
leptin receptor was detected. Importantly, the KATP channel subunits
Kir6.1 and SUR1 were also detected whilst neither Kir6.2 nor SUR2 mRNA was
found (Fig. 9Ba).
-tubulin
together with the known KATP channel subunits (Kir6.1, Kir6.2, SUR1
and SUR2) and the long form of the leptin receptor (Ob-Rb). In each
glucose-receptive neurone tested, mRNA for the three housekeeping genes and the
leptin receptor was detected. Importantly, the KATP channel subunits
Kir6.1 and SUR1 were also detected whilst neither Kir6.2 nor SUR2 mRNA was
found (Fig. 9Ba).
|
|
|
View larger version |
|
|
||
|
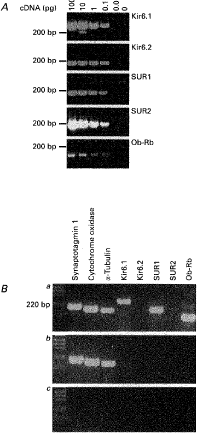
Figure 9.
Molecular identity of the KATP channel complex A, 2·5 % agarose gel showing the
sensitivity of the Kir6.1, Kir6.2, SUR1, SUR2 and leptin receptor primer
pairs. Ba, using the cytoplasmic contents harvested from a single
glucose-receptive VMH neurone, the expression of the KATP channel
subunits Kir6.1 and SUR1 together with the housekeeping genes synaptotagmin
1, cytochrome oxidase and |
||
In a parallel control experiment,
the cytoplasmic contents of five non-glucose-receptive VMH neurones were
harvested and after reverse transcription the cDNA was subjected to PCR
analysis using the same primer oligonucleotides as used on the
glucose-receptive neurones. As observed in the glucose-receptive neurones, the housekeeping
genes synaptotagmin 1, cytochrome oxidase and ![]() -tubulin
were detected. However although one of the five cells was found to express mRNA
for SUR2, none of the five cells was found to contain mRNA for the leptin
receptor or the KATP channel subunits present in the
glucose-receptive neurones (Fig. 9Bb).
-tubulin
were detected. However although one of the five cells was found to express mRNA
for SUR2, none of the five cells was found to contain mRNA for the leptin
receptor or the KATP channel subunits present in the
glucose-receptive neurones (Fig. 9Bb).
To confirm that this pattern of KATP
channel expression was indeed representative of the glucose-receptive neurones
under study, the cytosolic contents of four neurones identified as glucose
responsive using cell-attached recordings were also harvested and examined for
KATP channel expression. In this instance the KATP
channel subunits Kir6.1 and SUR1 were again detected whilst neither Kir6.2 nor
SUR2 mRNA was found (Fig.
10).
|
|
|
View larger version |
|
|
||
|
Figure 10.
Glucose-receptive neurones in the VMH express KATP channels
composed of Kir6.1 and SUR1 subunits A, cell-attached recording made at a
pipette potential of 0 mV. Removal of glucose from the bath led to the
reversible activation of a 3·7 pA channel which was inhibited by tolbutamide.
Leptin 22-56 mimicked the effect of glucose removal in a non-reversible
manner. Ba, using the cytoplasmic contents harvested from the same
glucose-receptive VMH neurone, the expression of the KATP channel
subunits Kir6.1 and SUR1 together with the housekeeping genes synaptotagmin
1, cytochrome oxidase and |
||
|
|
DISCUSSION |
|
|
Identification of
glucose-receptive neurones in the rat VMH
Using cell-attached patch
recordings, a subset of VMH neurones has been identified whose firing rates are
regulated by the concentration of extracellular glucose. This ability to
respond to extracellular glucose was found to be due to the presence of a sulphonylurea-sensitive
channel with characteristics similar to those found in the pancreatic ![]() cell.
In accordance with these findings, whole-cell recordings, performed with an
electrode solution containing no ATP, hyperpolarized a similar proportion of
VMH neurones through the activation of a sulphonylurea-sensitive outward
current possessing a reversal potential close to the calculated equilibrium
potential for potassium ions (approximately -100 mV). Since this current was
not observed when ATP was present in the electrode solution it is likely that
the outward sulphonylurea-sensitive current observed in this study is also ATP
sensitive. In a further series of experiments we were able to exploit the
ability to identify visually and hence re-patch individual neurones to confirm
that those cells identified as glucose responsive in cell-attached recordings
were synonymous with those hyperpolarized by intracellular dialysis with an
ATP-free solution.
cell.
In accordance with these findings, whole-cell recordings, performed with an
electrode solution containing no ATP, hyperpolarized a similar proportion of
VMH neurones through the activation of a sulphonylurea-sensitive outward
current possessing a reversal potential close to the calculated equilibrium
potential for potassium ions (approximately -100 mV). Since this current was
not observed when ATP was present in the electrode solution it is likely that
the outward sulphonylurea-sensitive current observed in this study is also ATP
sensitive. In a further series of experiments we were able to exploit the
ability to identify visually and hence re-patch individual neurones to confirm
that those cells identified as glucose responsive in cell-attached recordings
were synonymous with those hyperpolarized by intracellular dialysis with an
ATP-free solution.
Examination of the resting membrane
properties of these neurones immediately following formation of the whole-cell
configuration revealed characteristics similar to those previously associated
with glucose-receptive neurones in the guinea-pig VMH using sharp electrodes
(Minami et al. 1986) and those of rat type 3 VMH neurones previously
described by Priestley (1992).
Properties of the KATP
channel current
Under voltage-clamp conditions,
this KATP channel current was sensitive to the sulphonylureas
tolbutamide and glibenclamide and to the non-sulphonylurea moiety of
glibenclamide, meglitinide. The ability of these agents to inhibit the KATP
channel current profoundly at nanomolar and low micromolar concentrations together
with their irreversibility is similar to that seen for both the native and
cloned pancreatic ![]() cell
KATP channel complexes (Kir6.2 and SUR1; Lee et al. 1994a;
Gribble et al. 1997). Furthermore, this current also displays a similar
sensitivity to the imidazoline KATP channel inhibitor phentolamine,
and to the potassium channel opener diazoxide (Dunne, 1991). On the basis of
these findings, it would appear that the KATP channel complex
present in these neurones is pharmacologically similar to the complex present
in the
cell
KATP channel complexes (Kir6.2 and SUR1; Lee et al. 1994a;
Gribble et al. 1997). Furthermore, this current also displays a similar
sensitivity to the imidazoline KATP channel inhibitor phentolamine,
and to the potassium channel opener diazoxide (Dunne, 1991). On the basis of
these findings, it would appear that the KATP channel complex
present in these neurones is pharmacologically similar to the complex present
in the ![]() cell.
cell.
In agreement with this suggestion,
in cell-attached patch recordings, the unitary properties of the
sulphonylurea-sensitive channel were virtually indistinguishable from those
reported in the pancreatic ![]() cell.
Furthermore, single cell RT-PCR analysis demonstrated the co-expression of
Kir6.1 and SUR1 subunits in these neurones. Although this combination of KATP
channel subunits has not been observed in the periphery, we have recently found
that the same combination comprises the KATP channel in rat striatal
cholinergic interneurones (Lee et al. 1998). Interestingly the unitary
conductance of the two channels appears to differ in the two sets of neurones
raising the possibilty that other as yet unknown factors also contribute to the
properties of the native KATP channel complex.
cell.
Furthermore, single cell RT-PCR analysis demonstrated the co-expression of
Kir6.1 and SUR1 subunits in these neurones. Although this combination of KATP
channel subunits has not been observed in the periphery, we have recently found
that the same combination comprises the KATP channel in rat striatal
cholinergic interneurones (Lee et al. 1998). Interestingly the unitary
conductance of the two channels appears to differ in the two sets of neurones
raising the possibilty that other as yet unknown factors also contribute to the
properties of the native KATP channel complex.
It is noteworthy that the unitary
conductance of the Kir6.1 subunit has been demonstrated to differ when
expressed with different ABC transporter molecules. Thus when expressed alone,
this subunit exhibits a unitary conductance of 70 pS (Inagaki et al.
1995b) compared with 33 or 52 pS when expressed with SUR2B or cystic
fibrosis transmembrane conductance regulator (CFTR), respectively, (Yamada et
al. 1997; Ishida-Takahashi et al. 1998). Although these
discrepancies may be related to differences in experimental protocol it is also
feasible that ABC transporter molecules together with other as yet unknown
factors can affect the conductance properties of this KATP channel
subunit in a presently poorly understood manner.
Comparison with previous studies
These results differ considerably
from previous studies performed upon glucose-receptive neurones in this
nucleus. In these former studies, the channel was found to exhibit a much
larger unitary conductance (150 pS) than that reported here and was seemingly
unaffected by glibenclamide. In addition, this channel was found to be
insensitive to the potassium channel opener diazoxide (Sellars et al.
1992). At present we are unable to provide a definitive answer for these
discrepancies, although a number of potential reasons exist.
One possibility is that the former
and present studies were performed on the same KATP channel complex
but that the different experimental conditions employed produced an alteration
in the channel properties. With regard to this it is noteworthy that the
results obtained previously were from enzymatically dispersed neurones from the
VMH area. It is now well established that enzymes such as trypsin when applied
to the intracellular surface of the KATP channel complex alter both
the ATP and sulphonylurea sensitivity of these molecules (Lee et al.
1994b). However, this procedure has not previously been shown to alter
the unitary conductance of such channels and we consider that it is unlikely
for these proteases to have gained access to the intracellular surface of
neurones that have remained viable for electrophysiological analysis.
Additionally, a second group has used a similar enzymatic dispersal procedure
and obtained a glibenclamide sensitivity more akin to that obtained in the present
study (Routh et al. 1997). These authors suggest that the difference in
unitary conductance may be due to the channel adopting a lower subconductance
state in their studies. While this remains feasible, this suggestion cannot
account for the different pharmacological properties found in this study.
Another possibilty for these
discrepancies is that more than one type of glucose-receptive neurone exists
within the VMH. However, if this were the case, it is hard to reconcile why
this second type of glucose-receptive neurone was not identified in at least
some of the recordings performed in the present study. Since previous studies
have been performed on acutely dispersed neurones obtained from hypothalamic
slices containing the VMH (and also arcuate nucleus; M. L. J. Ashford, personal
communication) the precise location of this second class of glucose-receptive
neurone is not entirely known. It is therefore plausible that glucose-receptive
neurones in the VMH are composed of classical KATP channel subunits
whilst a second class of glucose-receptive neurone exists in hypothalamic
nuclei bordering the VMH containing a different type of KATP
channel. Furthermore, since former studies have been performed primarily on
excised patches, the rapid rate of run-down exhibited by the present channel
would immediately bias results in favour of the non-classical KATP
channel which does not run down.
This suggestion is supported by the
results of Routh et al. (1997) which were obtained using punch
dissections of the VMH. Using this procedure, a channel with characteristics
similar to those found presently was reported. In these studies, the authors
report that the channel was sensitive to micromolar concentrations of
glibenclamide although it is unclear whether lower concentrations were tested.
The glibenclamide-sensitive channel was also found to exhibit a rapid loss of
activity on patch excision due to run-down but this process was reversed by
phosphorylating conditions.
Physiological significance
It is now well established that the
VMH is an important satiety centre in the regulation of food intake. Lesions of
this nucleus are known to cause hyperphagia whilst electrical stimulation
suppresses food intake (Anand et al. 1964). Furthermore, various
neuromodulators of food intake are known to exert their effects at least partly
via the VMH (Morley & Levine, 1985). It is also well established that a
subset of VMH neurones are able to modify their electrical activity in response
to changes in the extracellular concentration of glucose (Ono et al.
1982). Recent studies have demonstrated that the ability of these neurones to
react to changes in blood glucose may be mediated by KATP channel
activity (Ashford et al. 1990a).
In the present series of
experiments we have confirmed these observations. However, the nature of the KATP
channel conductance observed in the present study contrasts significantly with
that obtained previously in the rat. In the present study we demonstrate that
the pharmacological and molecular biological properties of this channel are
essentially the same as those found for the KATP channel complex in
tissues such as the pancreatic ![]() cell
and mouse VMH (Rowe et al. 1998). Since we have been unable to
distinguish a second class of glucose-receptive neurone in this study, the
exact location and molecular make-up of these cells remain to be elucidated.
cell
and mouse VMH (Rowe et al. 1998). Since we have been unable to
distinguish a second class of glucose-receptive neurone in this study, the
exact location and molecular make-up of these cells remain to be elucidated.
In future studies it will be
important to examine the physiological and pathophysiological modulation of
this channel complex with a view to elucidating its potential for therapeutic
intervention. In relation to this it is interesting to note that both our
studies and those of other groups (Spanswick et al. 1997) indicate a
close and apparently exclusive relationship between the peptide hormone leptin
and glucose-receptive neurones in this area of the CNS. These findings support
the suggestion that these neurones perform an important role in the regulation
of food intake and indicate that it may be possible to modulate the activity of
these neurones therapeutically.
|
|
REFERENCES |
|
|
|
Anand, B. K., Chhina, G. S., Sharma, K. N., Dua, S. &
Singh, B. (1964). Activity of single neurons in the hypothalamic feeding
centres: effects of glucose. American Journal of Physiology 207,
1146-1154. |
|
|
Ashcroft, S. J. H. & Ashcroft, F. M. (1990). Properties and functions
of ATP-sensitive K+ channels. Cellular Signalling 2,
197-214 |
|
|
Ashford, M. L. J., Boden, P. R. & Treherne, J. M. (1990a).
Glucose-induced excitation of hypothalamic neurones is mediated by
ATP-sensitive K+ channels. Pflügers Archiv 415,
479-483 |
|
|
Ashford, M. L. J., Boden, P. R., Treherne, J. M. (1990b).
Tolbutamide excites rat glucoreceptive ventromedial hypothalamic neurones by
indirect inhibition of ATP-K+ channels. British Journal of
Pharmacology 101, 531-540 |
|
|
Dixon, A. K., Richardson, P. J., Lee, K., Carter, N. & Freeman, T. C.
(1998). Expression profiling of single cells using 3 prime end amplification
(TPEA) PCR. Nucleic Acids Research 26, 4426-4431 |
|
|
Dunne, M. J. (1991). Block of ATP-regulated potassium channels by
phentolamine and other adrenoceptor antagonists. British Journal of
Pharmacology 103, 1847-1850 |
|
|
Dunn-Meynell, A. A., Govek, E. & Levin, B. E. (1997). Intracarotid
glucose infusions selectively increase FOS-like immunoreactivity in
paraventricular and ventromedial nucleus neurons. Brain Research 748,
100-106 |
|
|
Grace, A. A. & Llinas, R. (1985). Morphological artifacts induced in
intracellularly stained neurons by dehydration; circumvention using rapid
dimethyl sulfoxide clearing. Neuroscience 16, 461-475 |
|
|
Gribble, F. M, Ashfield, R., Ämmälä, C. & Ashcroft, F. M. (1997).
Properties of cloned ATP-sensitive K+ currents expressed in Xenopus
oocytes. The Journal of Physiology 498, 87-98 |
|
|
Inagaki, N., Gonoi, T., Clement, J. P., Namba, N., Inazawa, J., Gonzalez,
G., Aguilar-Bryan, L., Seino, S. & Bryan, J. (1995a).
Reconstitution of IKATP: An inward rectifier subunit plus the
sulfonylurea receptor. Science 270, 1166-1170 |
|
|
Inagaki, N., Tsuura, Y., Namba, N., Masuda, K., Gonoi, T., Horie, M.,
Mizuta, M. & Seino, S. (1995b). Cloning and functional
characterization of a novel ATP-sensitive potassium channel ubiquitously
expressed in rat tissues including pancreatic islets, pituitary, skeletal
muscle and heart. Journal of Biological Chemistry 270,
5691-5694 |
|
|
Ishida-Takahashi, A., Otani, H., Takahashi, C., Washizuka, T., Tsuji, K.,
Noda, K., Horie, M. & Sasayama, S. (1998). Cystic fibrosis transmembrane
conductance regulator mediates sulphonylurea block of the inwardly rectifying
K+ channel Kir6.1. The Journal of Physiology 508,
23-30 |
|
|
Kozlowski, R. Z., Hales, C. N. & Ashford, M. L. J. (1989). Dual
effects of diazoxide on ATP-K+ currents recorded from an
insulin-secreting cell line. British Journal of Pharmacology 97,
1039-1050 |
|
|
Lee, K., Dixon, A. K., Freeman, T. C. & Richardson, P. J. (1998).
Identification of a KATP channel current in rat striatal
cholinergic interneurones. The Journal of Physiology 510,
441-453 |
|
|
Lee, K., Ozanne, S. E., Hales, C. N. & Ashford, M. L. J. (1994a).
Mg2+ dependent inhibition of KATP channels by
sulphonylureas in CRI-G1 insulin secreting cells. British Journal of
Pharmacology 111, 632-640 |
|
|
Lee, K., Ozanne, S. E., Rowe, I. C. M., Hales, C. N. & Ashford, M. L.
J. (1994b). The effects of trypsin on ATP-sensitive potassium channel
properties and sulfonylurea receptors in the CRI-G1 insulin-secreting cell
line. Molecular Pharmacology 45, 176-185. |
|
|
Minami, T., Oomura, Y. & Sugimora, M. (1986). Electrophysiological
properties and glucose responsiveness of guinea-pig ventromedial hypothalamic
neurones in vitro. The Journal of Physiology 380,
127-143 |
|
|
Morley, J. E. & Levine, A. S. (1985). The pharmacology of eating
behavior. Annual Review of Pharmacology and Toxicology 25,
127-146 |
|
|
Ono, T., Nishino, H., Fukuda, M., Sasaki, K., Muramoto, K.-I. &
Oomura, Y. (1982). Glucoresponsive neurons in rat ventromedial hypothalamic
tissue slices in vitro. Brain Research 232, 494-499 |
|
|
Powis, J. E., Bains, J. S. & Ferguson, A. V. (1998). Leptin
depolarises rat hypothalamic paraventricular nucleus neurons. American
Journal of Physiology 274, R1468-1472 |
|
|
Priestley, T. (1992). The effect of baclofen and somatostatin on neuronal
activity in the rat ventromedial hypothalamic nucleus in vitro. Neuropharmacology
31, 103-109 |
|
|
Routh, V. H., McArdle, J. J. & Levin, B. E. (1997). Phosphorylation
modulates the activity of the ATP-sensitive K+ channel in the
ventromedial hypothalamic nucleus. Brain Research 778, 107-119 |
|
|
Rowe, I. C. M., Spanswick, D. & Ashford, M. L. J. (1998). The
characterization of potassium channels present in glucose- and
sulphonylurea-sensitive hypothalamic neurones in isolation and in brain
slices of the mf1 mouse. The Journal of Physiology 506.P, 152P. |
|
|
Sakura, H., Ämmälä, C., Smith, P. A., Gribble, F. M. & Ashcroft, F. M.
(1995). Cloning and functional expression of the cDNA encoding a novel
ATP-sensitive potassium channel expressed in pancreatic |
|
|
Sellars, A. J., Boden, P. R. & Ashford, M. L. J. (1992). Lack of
effect of potassium channel openers on ATP-modulated potassium channels recorded
from rat ventromedial hypothalamic neurones. British Journal of
Pharmacology 107, 1068-1074. |
|
|
Spanswick, D., Smith, M. A., Groppi, V. E., Logan, S. D. & Ashford, M.
L. J. (1997). Leptin inhibits hypothalamic neurons by activation of
ATP-sensitive potassium channels. Nature 390, 521-525 |
|
|
Stuart, G. J., Dodt, H.-U. & Sakmann, B. (1993). Patch-clamp
recordings from the soma and dendrites of neurons in brain slices using
infrared video microscopy. Pflügers Archiv 423, 511-518 |
|
|
Sturgess, N. C., Hales, C. N., Cook, D. L. & Ashford, M. L. J. (1985).
The sulphonylurea receptor may be an ATP-sensitive K+ channel. Lancet
ii, 474-475. |
|
|
Yamada, M., Isomoto, S., Matsumoto, S., Kondo, C., Shindo, T., Horio, Y.
& Kurachi, Y. (1997). Sulphonylurea receptor 2B and Kir6.1 form a
sulphonylurea-sensitive but ATP-insensitive K+ channel. The
Journal of Physiology 499, 715-720 |
Corresponding author
K. Lee: Parke Davis Neuroscience
Research Centre, Cambridge University Forvie Site, Cambridge CB2 2QB, UK.
Email: kevin.lee@wl.com
This article has been cited by other articles:
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|||||
|