 Drug watch
Drug watch| Diabetologia Clinical and Experimental Diabetes and Metabolism |
| © Springer-Verlag 2005 |
| 10.1007/s00125-005-1707-5 |
 Drug watch
Drug watchDipeptidyl
peptidase inhibitors as new drugs for the treatment of type 2
diabetes
| (1) |
Lilly
Research Laboratories, Lilly Forschung, Essener Bogen 7,
22419 Hamburg, Germany |
| (2) |
Department
of Anatomy, University of Kiel, Kiel, Germany |
| H.-J. Mest Email: mest_hans-juergen@lilly.com Phone: +49-40-52724501 Fax: +49-40-52724617 |
Received: 31 December 2004 Accepted: 18 January 2005 Published online: 16 March 2005
Abstract Inhibitors of the regulatory protease dipeptidyl peptidase-IV (DPP-IV) are currently under development in preclinical and clinical studies (several pharmaceutical companies, now in Phase III) as potential drugs for the treatment of type 2 diabetes. Their development is based on the observation that DPP-IV rapidly inactivates the incretin hormone glucagon-like peptide-1 (GLP-1), which is released postprandially from the gut and increases insulin secretion. DPP-IV inhibitors stabilise endogenous GLP-1 at physiological concentrations, and induce insulin secretion in a glucose-dependent manner; therefore, they do not demonstrate any hypoglycaemic effects. Furthermore, they are orally bioavailable. In addition to their ability to protect GLP-1 against degradation, DPP-IV inhibitors also stabilise other incretins, including gastric inhibitory peptide and pituitary adenylate cyclase-activating peptide. They also reduce the antagonistic and desensitising effects of the fragments formed by truncation of the incretins. In clinical studies, when used for the treatment of diabetes over a 1-year period, DPP-IV inhibitors show improved efficacy over time. This finding can be explained by a GLP-1-induced increase in the number of beta cells. Potential risks associated with DPP-IV inhibitors include the prolongation of the action of other peptide hormones, neuropeptides and chemokines cleaved by the protease, and their interaction with DPP-IV-related proteases. Based on their mode of action, DPP-IV inhibitors seem to be of particular value in early forms of type 2 diabetes, either alone or in combination with other types of oral agents.
Keywords Dipeptidyl peptidase-IV - Glucagon-like peptide-1 - Incretin - Inhibitor - Insulinotropic effect - Novel drug - Side-effect - Type 2 diabetes
Abbreviations DPP-IV
dipeptidyl peptidase-IV - FAP fibroblast activation
protein-![]() - GIP gastric
inhibitory peptide - GLP-1 glucagon-like peptide-1 - PACAP
pituitary adenylate cyclase-activating peptide
- GIP gastric
inhibitory peptide - GLP-1 glucagon-like peptide-1 - PACAP
pituitary adenylate cyclase-activating peptide
Introduction
Dipeptidyl
peptidase-IV (DPP-IV) inhibitors are novel types of potential
drugs for the treatment of type 2 diabetes. The original concept
that inhibition of DPP-IV would improve glucose tolerance was
based on the observation that glucagon-like peptide-1 (GLP-1) is
rapidly cleaved and inactivated by the protease DPP-IV (see [1]
for detailed references). Inhibition of this proteolytic
inactivation should prolong the action of GLP-1, which is
released postprandially from the L-cells in the gut and increases
insulin secretion (the ![]() incretin
incretin![]() concept), resulting in improved glucose
tolerance. The possibility that other mediators may contribute to
these effects should not be excluded. Initial preclinical and
clinical studies (performed by several companies, now in Phase
III) on DPP-IV inhibitors have shown promising results [2,
3],
and the first inhibitors may reach the market within 3–4
years.
concept), resulting in improved glucose
tolerance. The possibility that other mediators may contribute to
these effects should not be excluded. Initial preclinical and
clinical studies (performed by several companies, now in Phase
III) on DPP-IV inhibitors have shown promising results [2,
3],
and the first inhibitors may reach the market within 3–4
years.
General
pharmacological value of DPP-IV inhibitors
Prior
to the discovery of DPP-IV inhibitors, it was known that patients
with type 2 diabetes have reduced levels of active GLP-1. This
may be at least partly responsible for impaired insulin
secretion, one of the major pathophysiological factors in the
development of type 2 diabetes. In fact, some therapies for type
2 diabetes are based on the enhancement of insulin secretion
through the action of sulphonylureas. Unfortunately, due to their
mode of action, which leads to insulin secretion in a
glucose-independent manner, treatment with this class of
insulinotropic agents can lead to hypoglycaemia and favour
obesity. It was initially postulated that inhibition of DPP-IV
would extend the half-life of endogenous GLP-1 and other
incretins known to induce insulin secretion from the beta cell in
a glucose-dependent manner. It was further proposed that the
glucose dependence of insulin secretion via this mechanism would
greatly decrease the risk of hypoglycaemia. In type 2 diabetic
patients, the amount of incretin released is not adequate for the
regulation of glucose homeostasis. Stabilisation of active GLP-1
via inhibition of DPP-IV might be sufficient to achieve a
physiological insulinotropic effect. The impaired insulin
secretion observed in type 2 diabetes is restored to the normal
level by the administration of exogenous GLP-1, as demonstrated
by the normalisation of the first phase of insulin secretion [4].
This differentiates the mechanism of action of DPP-IV/GLP-1 from
that of the sulphonylureas. In addition, DPP-IV inhibitors should
enhance the physiological role of other incretins, including
gastric inhibitory peptide (GIP) and pituitary adenylate
cyclase-activating peptide (PACAP) (Fig. 1).
The relative contributions of these two proteins to insulin
secretion are not yet fully understood. However, a recent study
by Ahrén and Hughes [5]
demonstrated the importance of the stabilisation of other
incretins by inhibitor treatment. When exogenous GLP-1 or
PACAP-38 is administered to mice in conjunction with a DPP-IV
inhibitor, the insulin response is increased by 75–80%
relative to that observed during treatment in the absence of
inhibitor. Similarly, in mice, the co-administration of a DPP-IV
inhibitor with GIP or gastrin-releasing peptide results in a
25–40% increase in the insulin response as compared with
that observed during treatment with the incretins alone.
Therefore, the treatment of type 2 diabetes patients with DPP-IV
inhibitors should help to restore impaired glucose homeostasis.
Furthermore, the glucose dependence of the insulin-releasing
effect of DPP-IV inhibitors should offer a significant advantage
over currently used insulin secretagogues.
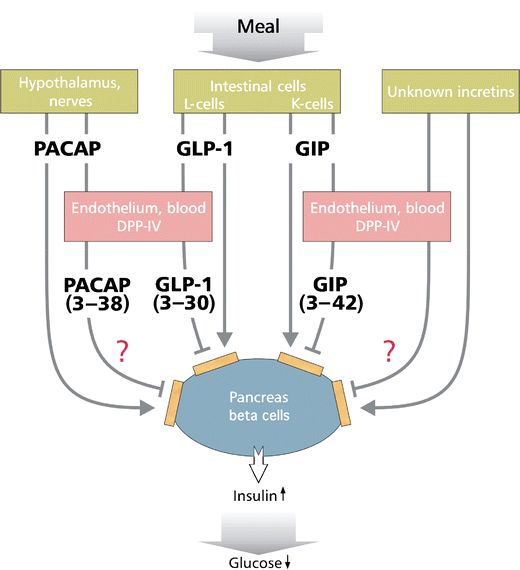
Fig. 1 Schematic drawing of the role of DPP-IV in the inactivation of incretins (GLP-1, GIP, PACAP) (see text). DPP-IV-truncated GLP-1 and GIP act as weak receptor antagonists
The time course of the pathophysiological processes associated with type 2 diabetes is not yet completely understood. It is generally agreed that these impairments are already present during an early phase of the disease. Consequently, the treatment of early phases of type 2 diabetes with DPP-IV inhibitors should be indicated. In addition to their use as a monotherapy during early phases of type 2 diabetes, combining DPP-IV inhibitors with existing medications could significantly improve current therapy. DPP-IV inhibitors could replace sulphonylureas, or at least strongly reduce the doses of sulphonylureas, thereby increasing the therapeutic window for these agents and decreasing the risk of hypoglycaemic events. Furthermore, DPP-IV inhibitors are not known to increase body weight; in fact, they may protect against obesity, as observed in DPP-IV knockout mice fed a high-fat diet [6].
The
effect of treatment with DPP-IV inhibitors on GLP-1
concentrations
Delayed
gastric emptying, nausea and vomiting are GLP-1-related
side-effects. These are seen with high, non-physiological
concentrations (above about 60 pmol/l in human plasma),
which are only achieved after exogenous administration of the
incretin. DPP-IV inhibition raises the proportion of active GLP-1
rather than the total GLP-1 concentration (up to 30 pmol/l
after a meal [7]).
In this way, inhibition supports the physiological role of GLP-1
without producing the GLP-1 concentrations that induce the
GLP-1-related side-effects.
Complex
mechanisms of DPP-IV inhibitor action
In
order to explain the insulinotropic effects of DPP-IV inhibitors,
mechanisms other than those that prevent GLP-1 degradation in the
circulation have to be taken into account. The stabilisation of
GLP-1 as a result of DPP-IV inhibition in the gastrointestinal
tract, and the effects of GLP-1 on sensory nerves (described by
Holst and Deacon in the present issue [8])
are plausible explanations. Furthermore, DPP-IV inhibitors
stabilise not only GLP-1, but also other insulinotropic hormones
and neuropeptides, as mentioned above (Fig. 1).
However, the presence of additional potential mediators of DPP-IV
inhibition does not necessarily diminish the role played by
GLP-1.
Indeed, some experimental studies have demonstrated reduced total GLP-1 secretion during treatment with a DPP-IV inhibitor. However, more important than the total concentration of GLP-1 are the proportions of active and inactive GLP-1. Only one-third to one-half of the postprandial GLP-1 in the plasma of healthy subjects and type 2 diabetic patients consists of active GLP-1, the rest being the inactive fragment formed by truncation [7]. During treatment with a DPP-IV inhibitor, the relative proportion of the active form is clearly increased [9].
DPP-IV-truncated GLP-1 and GIP act as receptor antagonists [10, 11], thereby decreasing the direct effects of native peptides and possibly desensitising the receptors (as observed after the DPP-IV truncation of chemokines [12, 13]). In other physiological systems, increased receptor stimulation/internalisation results in the compensatory upregulation of peptide synthesis/secretion. Such a feedback mechanism might occur in intestinal L-cells, but other more complex interactions between the different incretins are also possible (Fig. 1). Again, these feedback mechanisms might be influenced by the ratio of active/intact GLP-1, which is increased when DPP-IV is inhibited.
Slow
onset of insulinotropic effects of DPP-IV inhibitors
Both
short- and long-term (probably adaptive) insulinotropic effects
are observed during DPP-IV inhibition. In OGTTs following a
single administration, DPP-IV inhibitors show clear and immediate
effects in normal and diabetic animals [14].
Long-term diabetes treatment with these inhibitors results in
further improvement. For example, glucose tolerance in diabetic
patients was higher after the administration of inhibitors for 4
weeks than it was initially (see [2]).
In fact, HbA1c levels have been observed to be reduced
for at least 12 weeks with treatment (in a mixed application with
metformin [3]).
At present, the extent to which these long-term glucose-lowering
effects observed in humans can be explained by the prolonged
stabilisation of GLP-1 or other incretins is not clear. Treatment
with GLP-1 is well known to inhibit beta cell apoptosis and
induce beta cell proliferation in animals. The stabilisation of
other incretins or peptide factors via DPP-IV inhibition may have
a similar protective effect on beta cells [15].
Potential
risks associated with DPP-IV inhibitors
DPP-IV
is a pleiotropic enzyme that cleaves, and thereby generally
inactivates, a variety of peptide hormones, neuropeptides and
chemokines (reviewed in [16,
17]).
Furthermore, it acts as a binding protein for fibronectin and
adenosine deaminase, and is a co-stimulator of T-cell activation.
Whereas the latter physiological functions of DPP-IV are not
dependent upon its enzymatic activity, and therefore should not
be affected by inhibitors, they may well affect the regulatory
functions of DPP-IV.
In addition to stabilising the incretins GLP-1, GIP and PACAP, DPP-IV inhibitors also prolong the action of the hormones peptide YY and growth hormone-releasing hormone, the neuropeptides neuropeptide Y and substance P, and chemokines such as stromal cell-derived factor-1 (CXCL12) and macrophage-derived chemokine (CCL22). Potential side-effects resulting from the prolongation of action of these messengers include neurogenic inflammation (substance P, neuropeptide Y), increases in blood pressure (neuropeptide Y), enhanced general inflammation, and allergic reactions (chemokines). However, to date, such side-effects have not been observed in preclinical animal or clinical human studies [17].
Other
potential side-effects associated with DPP-IV inhibitors may
result from the inadvertent inhibition of related enzymes. DPP-IV
belongs to a group of serine proteases, of which the
physiological importance is largely unknown [18].
The enzymes most closely related to DPP-IV are fibroblast
activation protein-![]() (FAP, also termed
seprase), DPP-II (also termed DPP 7 or quiescent proline
peptidase), DPP 8, and DPP 9. Prolyl endopeptidase (also known as
prolyl oligopeptidase) and prolyl carboxypeptidase are less
closely related. With the exception of FAP, all are intracellular
(cytosolic or lysosomal) enzymes that are not involved in peptide
inactivation. Like DPP-IV, FAP is a cell-surface enzyme; however,
unlike DPP-IV, its expression is very restricted during
development. It is expressed in adults on activated fibroblasts,
and on a few types of cancer (melanomas, sarcoma cell lines,
reactive tumour stromal fibroblasts), although the precise
physiological functions of the enzyme are not known. DPP 8 and
DPP 9 are widely distributed cytosolic enzymes, and their
inhibition has been suggested to be responsible for at least some
of the toxic effects of DPP-IV inhibitors identified to date,
including alopecia, thrombocytopenia, anaemia, enlarged spleen,
multiple histological pathologies, and animal mortality [19].
Consequently a DPP-IV inhibitor should demonstrate high
specificity with respect to other peptidases mentioned above.
(FAP, also termed
seprase), DPP-II (also termed DPP 7 or quiescent proline
peptidase), DPP 8, and DPP 9. Prolyl endopeptidase (also known as
prolyl oligopeptidase) and prolyl carboxypeptidase are less
closely related. With the exception of FAP, all are intracellular
(cytosolic or lysosomal) enzymes that are not involved in peptide
inactivation. Like DPP-IV, FAP is a cell-surface enzyme; however,
unlike DPP-IV, its expression is very restricted during
development. It is expressed in adults on activated fibroblasts,
and on a few types of cancer (melanomas, sarcoma cell lines,
reactive tumour stromal fibroblasts), although the precise
physiological functions of the enzyme are not known. DPP 8 and
DPP 9 are widely distributed cytosolic enzymes, and their
inhibition has been suggested to be responsible for at least some
of the toxic effects of DPP-IV inhibitors identified to date,
including alopecia, thrombocytopenia, anaemia, enlarged spleen,
multiple histological pathologies, and animal mortality [19].
Consequently a DPP-IV inhibitor should demonstrate high
specificity with respect to other peptidases mentioned above.
Concluding
remarks
DPP-IV
inhibitors support the physiological role of GLP-1 by increasing
the proportion of the endogenous active form of the incretin.
Their effects are only needed postprandially, when GLP-1 is
released from L-cells in the gut. The resulting active GLP-1
levels are within the physiological range and do not reach the
pharmacological concentrations achieved by the exogenous
administration of GLP-1. Consequently, the side-effects
associated with the exogenous application of GLP-1/agonists have
not been observed and should not be expected. The reduction of
the antagonistic effects of GLP-1 fragments formed by truncation
of the incretin by DPP-IV also contributes to the insulinotropic
effects of these agents. Furthermore, DPP-IV inhibitors are
orally bioavailable and therefore offer greater benefits compared
with injectable agents in terms of patient compliance.
Conversely, the doses of GLP-1 injectable agents used induce
immediate pharmacological effects without risk of hypoglycaemia [20].
Given their related, but distinct, modes of action, GLP-1
agonists and DPP-IV inhibitors appear to complement one another,
suggesting that a combination of the two agents may be of
benefit.
In addition to GLP-1, DPP-IV cleaves other insulinotropic peptides, including GIP and PACAP; the influence of DPP-IV inhibitors on their physiological functions and interactions is not well understood. Nevertheless, the presence of other mediators of DPP-IV inhibition would not necessarily diminish the role of GLP-1. The long-term effects of DPP-IV inhibitors, especially in humans, await investigation. The potential risks associated with the stabilisation of other peptide hormones, neuropeptides and chemokines should also be considered, e.g. enhancement of inflammatory or allergic reactions. Increasing the specificity of the inhibitor for DPP-IV may reduce the side-effects caused by the inhibition of related enzymes.
Because DPP-IV inhibition primarily supports the physiological functions of endogenous GLP-1 and other insulinotropic hormones, it can be anticipated that such inhibitors will be of particular relevance in early forms of type 2 diabetes. Protective effects on beta cells would be of great value in these patients and might partly restore their impaired insulin secretion. Combination therapies with other types of oral agents, especially those enhancing the action of insulin on target cells (i.e. insulin sensitisers) should provide additional benefits and increased potencies.
Due to the fact that levels of GLP-1 secretion are lower than normal in obese individuals and type 2 diabetic patients, treatment with DPP-IV inhibitors may restore endogenous active GLP-1 to normal levels, thereby affording excellent therapeutic effects. In addition, if elevated GLP-1 levels result in increased beta cell mass in the long-term, treatment with DPP-IV inhibitors would offer great potential in the prevention, or even cure, of type 2 diabetes.
| 1. |
Mentlein
R (2005) Therapeutic assessment of GLP-1 agonists
compared with dipeptidyl peptidase IV inhibitors as
potential anti-diabetic drugs. Expert Opin Investig Drugs
14:57–64 |
| |
|
| 2. |
Ahrén
B, Simonsson E, Larsson H et al (2002) Inhibition of
dipeptidyl peptidase IV improves metabolic control over a
4-week study period in type 2 diabetes. Diabetes Care
25:869–875 |
| |
|
| 3. |
Ahrén
B, Gomis R, Standl E, Mills D, Schweizer A (2004) Twelve-
and 52-week efficacy of the dipeptidyl peptidase IV
inhibitor LAF237 in metformin-treated patients with type
2 diabetes. Diabetes Care 27:2874–2880 |
| |
|
| 4. |
Brenner
MB, Gromada L, Efanov AM, Bokvist K, Mest H-J (2003)
Restoration of first-phase insulin secretion by the
imidazoline compound LY374284 in pancreatic islets of
diabetes db/db mice. Ann NY Acad Sci 1009:332–340 |
| |
|
| 5. |
Ahrén
B, Hughes TE (2004) Inhibition of DPP-4 augments insulin
secretion in response to exogenously administered GLP-1,
GIP, PACAP and GRP in mice. Endocrinology (in press) |
| |
|
| 6. |
Conarello
SL, Li Z, Ronan J et al (2003) Mice lacking dipeptidyl
peptidase IV are protected against obesity and insulin
resistance. Proc Natl Acad Sci U S A 100:6825–6830 |
| |
|
| 7. |
Vilsbøll
T, Krarup T, Deacon CF, Madsbad S, Holst JJ (2001)
Reduced postprandial concentrations of intact
biologically active glucagon-like peptide 1 in type 2
diabetic patients. Diabetes 50:609–613 |
| |
|
| 8. |
Holst
JJ, Deacon CF (2005) Glucagon-like peptide-1 mediates the
therapeutic actions of DPP-IV inhibitors. Diabetologia
DOI 10.1007/S00125-005-1705-7 |
| |
|
| 9. |
Kieffer
TJ, McIntosh CH, Pederson RA (1995) Degradation of
glucose-dependent insulinotropic polypeptide and
truncated glucagon-like peptide 1 in vitro and in vivo by
dipeptidyl peptidase IV. Endocrinology 136:3585–3596 |
| |
|
| 10. |
Knudsen
LB, Pridal L (1996) Glucagon-like peptide-1-(9–36)
amide is a major metabolite of glucagon-like
peptide-1-(7–36) amide after in vivo administration
to dogs, and it acts as an antagonist on the pancreatic
receptor. Eur J Pharmacol 318:429–435 |
| |
|
| 11. |
Gault
VA, Parker JC, Harriott P, Flatt PR, O |
| |
|
| 12. |
Ludwig
A, Schiemann F, Mentlein R, Lindner B, Brandt E (2002)
Dipeptidyl peptidase IV (CD26) on T cells cleaves the CXC
chemokine CXCL11 (I-TAC) and abolishes the stimulating
but not the desensitizing potential of the chemokine. J
Leukoc Biol 72:183–191 |
| |
|
| 13. |
Proost
P, Schutyser E, Menten P et al (2001) Amino-terminal
truncation of CXCR3 agonists impairs receptor signaling
and lymphocyte chemotaxis, while preserving
antiangiogenic properties. Blood 98:3554–3561 |
| |
|
| 14. |
Pederson
RA, White HA, Schlenzig D, Pauly RP, McIntosh CH, Demuth
HU (1998) Improved glucose tolerance in Zucker fatty rats
by oral administration of the dipeptidyl peptidase IV
inhibitor isoleucine thiazolidide. Diabetes
47:1253–1258 |
| |
|
| 15. |
Pospisilik
JA, Stafford SG, Demuth HU et al (2002) Long-term
treatment with the dipeptidyl peptidase IV inhibitor
P32/98 causes sustained improvements in glucose
tolerance, insulin sensitivity, hyperinsulinemia, and
beta-cell glucose responsiveness in VDF (fa/fa) Zucker
rats. Diabetes 51:943–950 |
| |
|
| 16. |
Mentlein
R (1999) Dipeptidyl-peptidase IV (CD26)—role in the
inactivation of regulatory peptides. Regulatory Pept
85:9–24 |
| |
|
| 17. |
Mentlein
R (2004) Cell surface peptidases. Int Rev Cyt
235:165–213 |
| |
|
| 18. |
Rosenblum
JS, Kozarich JW (2003) Prolyl peptidases: a serine
protease subfamily with high potential for drug
discovery. Curr Opin Chem Biol 7:496–504 |
| |
|
| 19. |
Lankas
G, Leiting B, Roy RS et al (2004) Inhibition of DPP8/9
results in toxicity in preclinical studies: potential
importance of selective dipeptidyl peptidase IV
inhibition for the treatment of type 2 DM. Diabetes
53(Suppl 2):A2 |
| |
|
| 20. |
Degn
KB, Brock B, Juhl CB et al (2004) Effect of intravenous
infusion of exenatide (synthetic exendin-4) on
glucose-dependent insulin secretion and counterregulation
during hypoglycaemia. Diabetes 53:2397–2403 |
| |
|