|
|
|
|||||
|
Science, Vol 306, Issue 5693, 34-37 , 1 October 2004
|
[DOI:
10.1126/science.306.5693.34]
DIABETES:
Islet Transplants Face Test of Time
Jennifer Couzin
Will the Edmonton protocol, hailed as a major step toward a cure for type I
diabetes, hold up in the long run?
Ellen Berty was
driving home from her special-education job when the call came, on the cell
phone she'd bought expressly for this purpose. The caller spoke the magical
words every person needing a transplant dreams of hearing: "We have a
match." In her Mazda convertible, Berty let out a yell of triumph.
"I'd won the contest of my life," she recalls thinking on that sunny
June day 3 years ago.
Ten hours later, Berty lay sedated in a radiology suite at the National Institutes
of Health (NIH) in Bethesda, Maryland, while doctors delicately injected a
yellowish green solution into a vein feeding into her liver. The mix held
hundreds of thousands of islets, cells from the pancreas of a man who'd died
suddenly. These cells were supposed to supply Berty with the critical hormone
insulin she'd lacked for 40 years, ever since being diagnosed with type I
diabetes at the age of 13.
Berty's islet-cell transplant is part of a vast global experiment, a test of
a therapy that's been hailed as the greatest hope for curing type I diabetes.
Five years after physicians in Edmonton began transplanting islets under a new
and widely celebrated protocol, the long-term results of this strategy are
beginning to emerge. They paint a nuanced and still unfinished picture of a
treatment that some doctors concede is riskier than they expected and less
effective than they had hoped.
The NIH trial in which Berty enrolled reflects the promise and peril of
these transplants. Berty has been one of the lucky ones. She stayed off insulin
injections for 2 years after her transplant. Today, she's back on a low dose,
but she has relatively few side effects from the immunosuppressive drugs she
takes to prevent islet rejection. Like most islet recipients, Berty also has
none of the diabetes complications she suffered before.
Still, Berty was NIH's last islet-transplant patient. After treating her and
five others, NIH stopped accepting new volunteers, its physicians increasingly
anxious that antirejection drugs, which must be taken for life, were spawning
problems worse than those the transplanted islets were solving.
Other centers disagreed. They continued testing the procedure, and today
more than 300 patients have received islets under the protocol crafted by the
Edmonton team. NIH, the Juvenile Diabetes Research Foundation (JDRF), other
nonprofit organizations, and several European governments have poured hundreds
of millions of dollars into coaxing these transplants to work. But as islet
transplants expand and less experienced centers launch islet programs, it's
become less clear what "work" really means.
|
CREDIT: CHRIS MADDALONI |
The original goal of islet transplants has been met:
Lifelong diabetics receiving new islets have been able to abandon, at least for
a time, insulin shots. According to an NIH survey published last month, 22 of
38 islet recipients were still off insulin a year after their transplant. Those
numbers sag with time, though, and it's not known how long transplanted islets
can thrive, or what's killing them when they fail.
A more pressing question is whether
insulin independence is enough. A sizable minority of islet recipients struggle
with new health problems, from painful mouth ulcers to anemia to kidney
disease, largely attributed to the combination of antirejection drugs
prescribed by the Edmonton protocol. And no one knows whether patients given
islets actually live longer than they would have without them. A controversial
study from some of the NIH scientists who treated Berty hints that the risk of
a shortened life span might be real.
Physicians are launching clinical
trials to improve the safety and effectiveness of islet transplants, but
they're far from offering this experimental therapy to all but the most
severely affected diabetes patients. For one, there aren't enough cadaver
pancreases to go around. Although many are looking at stem cells as a renewable
source of islets, that's still a distant prospect.
Aldo Rossini, director of the
diabetes division at the University of Massachusetts Medical School in
Worcester, compares the current state of islet transplants to the Wright
brothers' first flight. "They flew a couple hundred feet"--a
remarkable accomplishment at the time, he notes. Still, says Rossini, "no
one could have expected us to fly to California in that plane."
Measures of success
Since 1972, when Paul Lacy, a researcher at Washington University in St. Louis,
cured diabetic rats by giving them healthy islets, transplanters have sought to
extend that success to humans. The approach seemed obvious: In type I diabetes,
the body's immune system mistakenly attacks insulin-producing islet cells in
the pancreas, and by the time the symptoms of diabetes surface, most of these
islet cells are gone. But in more than 400 human islet transplants beginning in
the 1970s, doctors couldn't get transplanted cells to stick. Many suspected
that, ironically, the steroid drugs given to prevent islet rejection were also
toxic to islet cells.
Then in the summer of 2000, the
dreary world of islet transplants changed forever. A team at the University of
Alberta in Edmonton, Canada, reported in The New England Journal
of Medicine that they'd given islets to seven diabetes patients under a
new regimen, and after roughly a year, all seven were still off insulin.
Unlike earlier islet transplants,
the Edmonton protocol didn't involve steroids. Led by James Shapiro, the
Edmonton team combined three antirejection drugs, one of which, sirolimus, had
recently begun human testing. It also gave patients islet cells from multiple
pancreases.
The group's report instantly became
medical legend. "Here," says David Nathan, director of the diabetes
center at Massachusetts General Hospital in Boston, "was this absolute
miracle."
Research funders quickly responded
to Edmonton's success. JDRF, one of the country's wealthiest and most powerful
disease advocacy groups, declared islet transplants a top priority, and since
2000 it has poured $225 million into the field. Hospitals in the United States
and Europe raced to set up islet-transplant centers, and patients flocked to
them in droves. Emory University's 18-month-old islet-transplant program has
fielded 5500 inquiries from patients, says surgeon Christian Larsen, its
director. Constrained by strict entry criteria and a tight budget, Emory has
given transplants to just six.
Like others in the field, Larsen
believes that ideal islet-transplant candidates are patients who, despite their
best efforts, cannot control their blood sugar. More dangerously, their bodies
have lost the ability to sense blood sugar lows, resulting in sudden fainting
spells, seizures, and even comas or death. For patients like Berty, who
suffered middle-of-the-night seizures and blackouts while driving, the
condition is terrifying and profoundly disruptive. It's these patients--maybe
1% of type I diabetics--who islet transplanters welcomed into clinical trials.
"Every patient we take on, they're near death's door or in desperate
straits," says Shapiro.
|
CREDIT: K. SUTLIFF/SCIENCE |
Transplanters
quickly found, however, that the success of the Edmonton protocol is tough to
sustain; the new islets seem to fade over time. Experienced islet-transplant
centers like Edmonton, the University of Miami, and the University of
Minnesota, Twin Cities, boast insulin independence rates of 80% to 90% a year
after transplant, far higher than the rates of many smaller centers. After 3
years, that falls to 60% among Miami's patients, says Camillo Ricordi,
scientific director of the Diabetes Research Institute there. Mark Atkinson, a
pathologist who studies diabetes at the University of Florida, Gainesville, and
research chair of JDRF, recently reviewed unpublished data on patients from
Edmonton, 3 to 4 years after their transplants. Between 12% and 25% were
insulin independent, he says. Among the original Edmonton seven, only two
remain off insulin, says Shapiro.
"Something is not going in the
right direction long term," says Ricordi. One possibility, he says, is
that the antirejection drugs, although less toxic to islets than steroids,
still harm the cells. Some nondiabetic patients taking the drugs after
receiving liver, heart, or kidney transplants have developed diabetes, notes
David Sutherland, chief of transplantation at the University of Minnesota.
A more fundamental problem may be
that the immunosuppressive drugs can't erase the underlying autoimmune response
that killed a patient's original islets. "These people don't like islets,
no matter whose they are," says Peter Senior, an endocrinologist at the
University of Alberta.
Another explanation for islet
failure is that patients may be receiving too few islets, even if they get
cells from multiple donors. A normal pancreas has roughly 1 million islets, but
current techniques allow only about 400,000, at most, to be extracted from a
donor pancreas. Moreover, unknown numbers die soon after they're transplanted,
forcing the rest to labor unusually hard to supply enough insulin. The islet
cells may just "poop out" over time, says Sutherland.
Edmonton found that giving patients
islets from as many as three pancreases could sustain insulin production
longer. But pancreases are a scarce and costly resource. Fewer than 2000 are
donated each year, and most go toward whole-organ pancreas transplants for
diabetes. In the United States, they also cost from $15,000 to $25,000 each.
Increasingly, however,
transplanters are wondering whether insulin independence, a goal pushed heavily
by islet-transplant centers, funders, and many patients, is the only yardstick
by which to measure islet-transplant success. Patients like Ellen Berty and
others who have gone back on insulin have found that partial islet function can
stave off the hypoglycemia they experienced before their transplants. This has
doctors hoping that islet transplants might prevent long-term complications of
diabetes, even if recipients still need some insulin. "Even if they're not
off insulin," says Shapiro, "their problems go away."
Walking a tightrope
But what if the therapy is as bad as the disease? Last month, the risky nature
of these transplants was underscored by NIH's first report from its
Collaborative Islet Transplant Registry. None of the 86 islet recipients NIH
surveyed died from the procedure. But the agency cataloged 20 serious adverse
events linked to islet transplants. They include four cases of life-threatening
neutropenia, a depletion of white blood cells caused by antirejection drugs.
"Islet transplants are still incredibly experimental," says Ricordi.
Amy Parker learned that the hard
way. Parker, who asked that her real name not be used, was diagnosed with type
I diabetes as a teenager. As her disease became progressively more unmanageable,
she began having seizures from low blood sugar, and blood vessels behind her
eyes started to leak. She needed multiple laser eye surgeries to preserve her
vision.
In 1999, soon after Edmonton began
its revolutionary set of islet transplants for patients like her, she applied.
In November and December 2002, Parker underwent two separate islet transplants.
Then, her new ordeal began.
|
CREDIT: COURTESY OF OFFICE OF PUBLIC
AFFAIRS/UNIVERSITY OF ALBERTA |
Since receiving
the transplants, her insulin requirements have dropped to a quarter of what
they once were, and she no longer suffers seizures or hypoglycemia. But every
day she experiences "deathly horrible" headaches, a result of the
antirejection drugs, she learned. Two summers ago, she began having trouble
breathing while on a family vacation in British Columbia. In July, she was
switched from the drug sirolimus, a possible culprit, to mycophenolate, another
immunosuppressant. If that fails to help her, says Parker, she may drop out of
the study and lose her islets.
The experimental nature of islet
transplants was further driven home last June at the American Diabetes
Association meeting in Orlando, Florida, where the Edmonton team released
troubling kidney function data on its first 45 islet-transplant patients. Of
the five patients Edmonton has followed for 4 years, two have "quite bad
renal outcomes," including one who has required dialysis, says Senior.
Overall, a third of the 45 have high levels of a protein in their urine that's
normally a harbinger of declining kidney function.
On the other hand, about a fifth of
diabetes patients typically develop kidney disease. Says Senior, "These
people may well have ended up with kidney failure irrespective of transplant.
The question is, are these drugs hastening that?"
Changing course
It's mixed news like this that has dampened enthusiasm among a handful of
doctors who once believed islet transplants were ready for patients. One is
David Harlan, a diabetes specialist at the National Institute of Diabetes and
Digestive and Kidney Diseases (NIDDK) in Bethesda, Maryland, who treated Berty.
Like his colleagues around the world, Harlan was enthralled by the Edmonton
protocol when it first appeared. In late 2000, he pulled together a transplant
team and more than $1 million in NIH funding to launch an islet- transplant
program at NIH. From 29 December 2000 through 14 June 2001--the date of Berty's
transplant--he and his colleagues performed transplants in six women with
severe type I diabetes.
The team quickly grew troubled by
what it was seeing. Looked at through the lens of diabetes, the picture was
relatively rosy: Four of six patients became insulin independent, and three
stayed that way for at least a year and a half. Even those who still needed
some insulin no longer suffered the hypoglycemic episodes that had driven them
to this experimental trial in the first place.
But problems abounded. Two
patients, including one off insulin, had to discontinue immunosuppressants
because of the intolerable side effects, such as deteriorating kidney function,
and their bodies rejected the islet cells. Even Ellen Berty, the NIH success
story, ran into some trouble. In her first year after the transplant, the
antirejection drugs contributed to a severe foot infection and caused mouth
ulcers so large that NIH dentists photographed them for use in a textbook. For Harlan,
the price NIH islet recipients were paying didn't seem worth it.
"When you expand the
experience, you find problems that were not expected," says Antonio
Secchi, head of the transplant program at Milan's University Vita-Salute San
Raffaele, one of about four major European islet-transplant centers. Two of his
center's 10 islet recipients who became insulin independent have since dropped
out of the program because of drug side effects.
One central question that
preoccupies Harlan is whether islet recipients will live longer than those in
comparable health who don't receive transplants. It's too early to answer that
question directly, so Harlan turned to data on pancreas transplants. They have
been used for years in much the way islet transplants are now, although most
are given to diabetes patients who also need kidneys.
Harlan and his colleagues examined
data from 124 transplant centers in the United States from 1995 to 2000 and
arrived at an unsettling conclusion: Patients receiving a solitary pancreas or
a pancreas after a kidney transplant were more likely to die within 4 years
than those still on the waiting list.
Published last December in the Journal
of the American Medical Association, the article touched off a furor. Many
transplant surgeons disputed its results. Minnesota's Sutherland and his
colleague Rainer Gruessner have reanalyzed the data, and Sutherland says
they've arrived at a conclusion opposite to Harlan's. Some patients in Harlan's
study, says Sutherland, were on the waiting list of more than one hospital and
ended up being counted twice. The study also excluded patients awaiting
pancreas transplants who had very poor kidney function; Harlan worried that
that might produce misleading results, but Sutherland believes those patients
should be included.
Concerns about long-term survival
after an islet transplant, however, must be weighed against the improved
quality of life that many transplant recipients experience, at least initially.
"The psychological benefit of insulin independence is potentially
enormous," says Emory's Larsen, "and it's hard to understand for a
nondiabetic."
Rita Hart, 46, is off insulin after
undergoing three transplants at Miami over 2 years, the last in July 2003.
Before her transplant, diabetes was consuming her life and complications were
piling up. "I was losing hope," she says. Now, despite drug side
effects that include anemia, she feels vastly more optimistic.
"It's striking how many
patients ask for a third transplant," says Senior. "Even with all the
side effects and all the downsides, they still think it's a good thing."
|
CREDIT: MANFRED KAGE/PETER ARNOLD |
And so
Edmonton, like many other islet-transplant centers, continues to grow. Today,
more than 25 hospitals have performed islet transplants that hew closely to the
Edmonton protocol. NIH will soon announce $75 million in awards for a new
clinical islet transplantation consortium in which centers will collaborate on
islet studies. Although Harlan ended his islet-transplant trial early, the
agency believes the treatment is worth pursuing. "This is not a
black-and-white issue," says Allen Spiegel, director of NIDDK.
Roadblocks to expansion
New money, however, will go only so far: Islet transplants are extraordinarily
expensive, costing up to $200,000 in the United States for one patient in the
first year. Antirejection drugs add another $30,000 annually after that. At
centers like Miami, where most patients remain part of a protocol, the price of
success--of supporting patients for years after a transplant--is becoming
prohibitive, says Rodolfo Alejandro, an endocrinologist and director of the
clinical islet-transplant program at the University of Miami. (Costs in Canada
are somewhat lower because there's no charge for organs, and the Alberta health
care system agreed in 2001 to pay for transplants for Alberta residents.)
Because they're still considered experimental, most United States islet
transplants are funded by NIH, JDRF, and sometimes by pharmaceutical companies
that manufacture immunosuppressants.
Costs are one roadblock to
performing the kind of large, controlled studies that some say are needed
before islet transplants can shift from being an experimental therapy to being
one approved by the U.S. Food and Drug Administration (FDA). Some islet
transplanters, like Alejandro, believe that one option is for FDA to approve
the therapy under its existing "orphan drug" category, making it
available to essentially the same patients getting islets now--those with
uncontrolled diabetes. That way, it could be covered by insurance. A year ago,
FDA held a public advisory committee meeting in Gaithersburg, Maryland, and
agency officials made clear they want certain issues addressed first. Those
include consistency in how islets are processed and a better assessment of the
risk-benefit balance.
No matter how FDA rules, major
hurdles stand in the way of islet transplants going mainstream. First, the
shortage of donor pancreases means scientists must find a renewable source of
islets. One popular option would involve using some type of stem cell. This
year, JDRF has committed more than $8 million to stem cell research, more than
$6 million of it to human embryonic stem cell work. Yet creating islets from
stem cells isn't imminent, according to Larsen and other transplanters.
Milder immunosuppressive regimens
might come more rapidly. One study that's gearing up at Miami calls for giving
islet recipients a dose of bone marrow cells culled from the donor's vertebrae,
to try to help patients better tolerate the islet cells.
Current islet recipients, and the
many more people with diabetes hoping for a transplant, are eagerly awaiting
the day when islet transplants are easier to come by and gentler to receive.
But Berty remains upbeat. A book she's written chronicling her experience came
out this spring. Its title: I Used to Have Type 1 Diabetes: Kiss My Islets.
 All smiles, in this
case. Ellen Berty and her NIH doctor David Harlan both say her islet
transplant was a success. But Harlan worries that not everyone has been so
lucky.
All smiles, in this
case. Ellen Berty and her NIH doctor David Harlan both say her islet
transplant was a success. But Harlan worries that not everyone has been so
lucky. 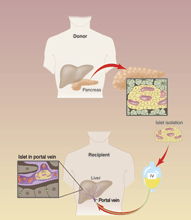
 Believer.
James Shapiro pioneered the Edmonton protocol, in which more than 300
patients with diabetes have participated.
Believer.
James Shapiro pioneered the Edmonton protocol, in which more than 300
patients with diabetes have participated. 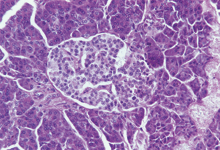 Donation in demand.
Islet cells such as these are in short supply for transplants.
Donation in demand.
Islet cells such as these are in short supply for transplants.